39+ calculate the atomic mass of element x
It is expressed in unified atomic mass units represented by the unit symbol u. The following conversion factors are used in the Atomic Mass Calculator.

Three Elements Of Dobereiner S Triad Are X Y Z If The Atomic Mass Of X Is 7 And Z Is 39 Calculate The Atomic Mass Of Y
Then you do the mass number multiplied by its percentage and add those two together and youll get the atomic mass of that element.
. 341 Number of neutrons rounded mass number atomic number Atoms of the element chromium Cr have an atomic number of 24 and a mass number of 52. It has the mass of three subatomic particles such as proton electron and neutron. The masses amu and abundances of the isotopes are given in the table below.
The mass numbers of its isotopes the abundance of these isotopes. Web Because most elements exist as mixtures of several stable isotopes the atomic mass of an element is defined as the weighted average of the masses of the isotopes. In a weighted average we multiply each value by a number representing its relative importance.
If you want to calculate how many neutrons an atom has you can simply subtract the number of protons or atomic number from the mass number. Web The atomic mass is defined as the massweight of the single atom of a chemical element. Isotope Abundance 221x 82900 220x 13200 218x 39000 Mass 22090 22000 21810 This problem has been solved.
A property closely related to an atoms mass number is its atomic mass. And for protium lets look at protium here. 75 of all X atoms are X-28 which means that 25 of all X atoms are X-24 as there are only 2 isotopes.
Web Atomic Mass Formula. This value on a periodic table is given in atomic mass units or amu but for chemistry calculations you usually write atomic mass in terms of grams per mole or gmol. For example naturally occurring carbon is largely a mixture of two isotopes.
Web The atomic mass or atomic weight is the decimal number The number of significant figures varies according to the table but the value is around 1201. 9889 12 C mass 12 amu by definition and 111 13 C mass 13003355 amu. In this problem the percent abundance represents the relative importance of each isotope.
If there are more isotopes just use the same method for all of the isotopes. So A is equal to Z plus N. Web Knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction.
Web The element X has three naturally occurring isotopes. Web Together the number of protons and the number of neutrons determine an elements mass number. Mass number protons neutrons.
The average relative atomic mass of element X is 2204. Web The mass number is the combined number of protons and neutrons in a nucleus so its protons and neutrons and its symbolized by A. In general one atomic mass is equal to the 112 of the mass single carbon -12 atom.
The average atomic mass of the element is amu. For an element relative atomic mass is the average mass of the naturally. 1 u 166054 10 -27 kg.
If masses are expressed in kilogram kg the result will be displayed in kilograms. So A is the mass number which is equal to the number of protons thats the atomic number which we symbolized by Z plus the number of neutrons. Web 1 2 3 4 5 Calculating relative atomic mass - Higher The relative atomic mass Ar of an element is calculated from.
The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. Atomic mass in an atom or group of an atom is the sum of the masses of protons neutrons and electrons. Web The atomic mass is the weighted average of the atomic masses of each isotope.
When the mass of proton and neutron are expressed in atomic mass unit u the result is displayed in atomic mass unit u.

Variation Of The Dr Cross Section Of Pd Like W Resulting From Various Download Scientific Diagram

The Average Atomic Mass Of A Sample Of An Element X Is 16 2 U What Are The Percentages Of Isotopes 8 16x And 8 18x In The Sample

Atomic Weight Of An Element Is 30 Its Equivalent Wt Is 10 The Valency Of Element Will Be
What S Better Than The Mendelev Table And Why Quora
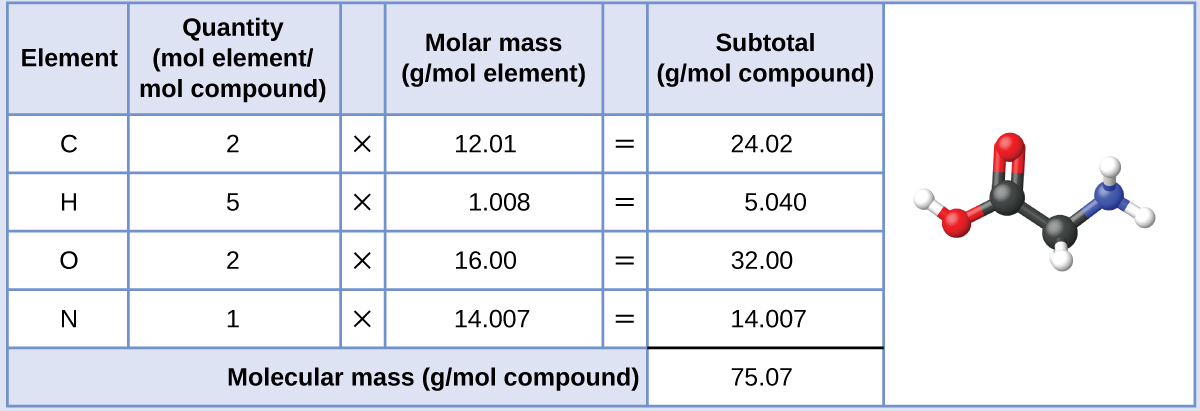
2 3 Calculating Atomic Masses Chemistry Libretexts

Pdf Relativistic Multireference Many Body Perturbation Theory Calculations On F Ne Na Mg Al Si And P Like Xenon Ions

Solved 1 20pts The Element Gd Has An Average Atomic Mass Chegg Com
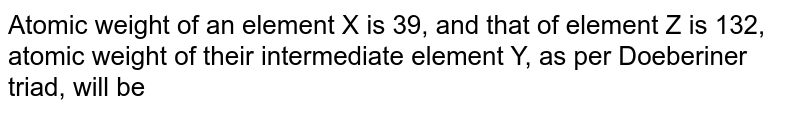
Three Elements Of Dobereiner S Triad Are X Y Z If The Atomic Mass Of X Is 7 And Z Is 39 Calculate The Atomic Mass Of Y

The Relative Atomic Mass Of An Element A Is 16 2 There Are Two Isotopes16 8 A And 18 8 A Of The Brainly In
Application Of Accelerators And Storage Rings Springerlink

Two Elements X And Y Atomic Mass Of X 75 And Y 16 Combine To Give A Compoun Youtube

Numerical Chemistry Pdf

Element X Contains Two Isotopes 78 X 19 5 And 80 X 80 5 What Is The Relative Atomic Mass Of Element X Quora

Solved Calculate The Atomic Mass Of Element X If It Has 2 Chegg Com

The Atomic Mass Of An Element X Is 16 24u What Are The Percentages Of Isotopes Of X Having Atomic No Brainly In

2 3 Calculating Atomic Masses Chemistry Libretexts
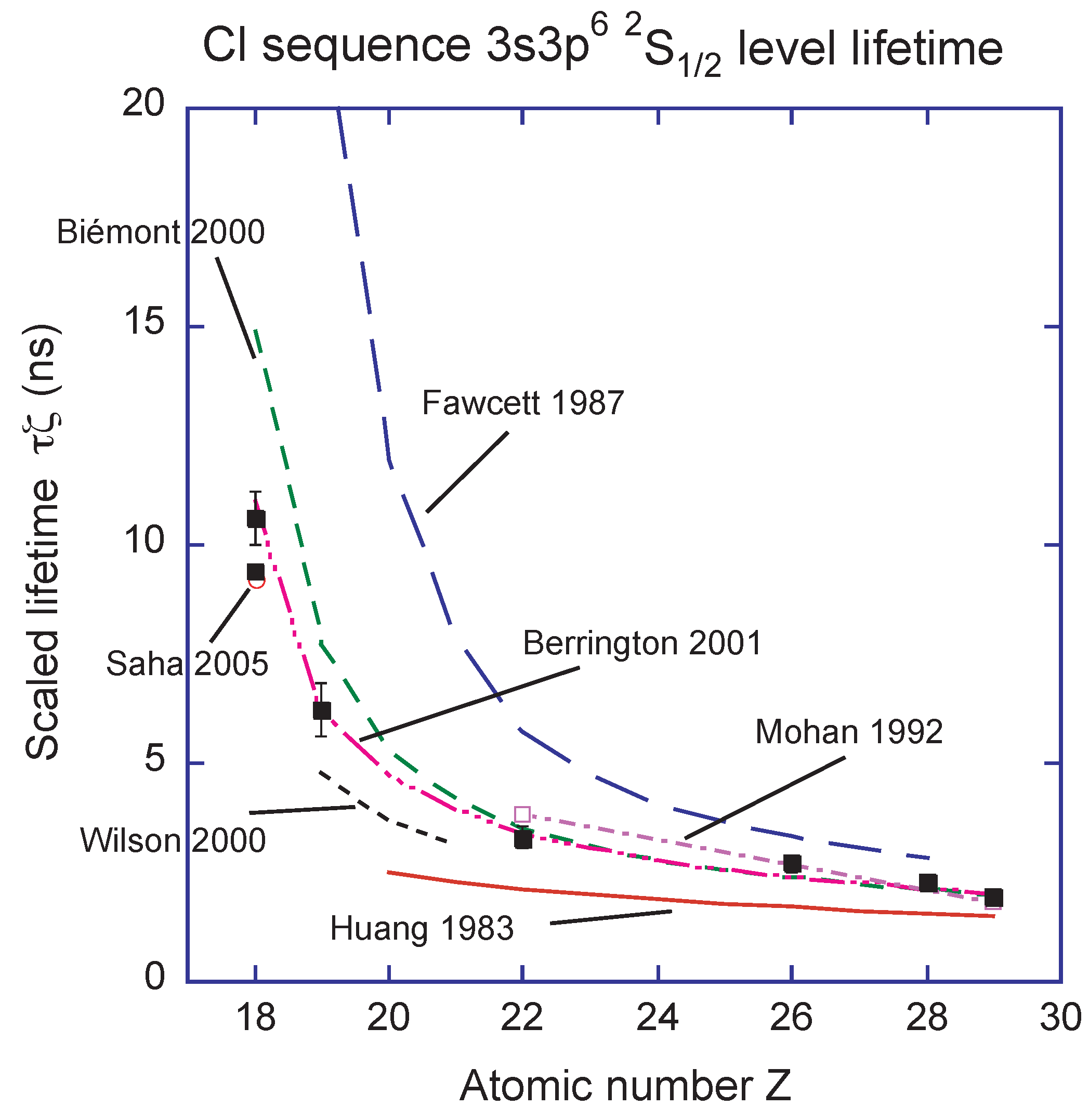
Atoms Free Full Text Critical Assessment Of Theoretical Calculations Of Atomic Structure And Transition Probabilities An Experimenter S View